0512-67998889(Suzhou)
18051093220(Shenzhen)
Molecular Cancer | CAR-M Therapy for Pancreatic Cancer Achieves Positive Results, Safe and Effective!
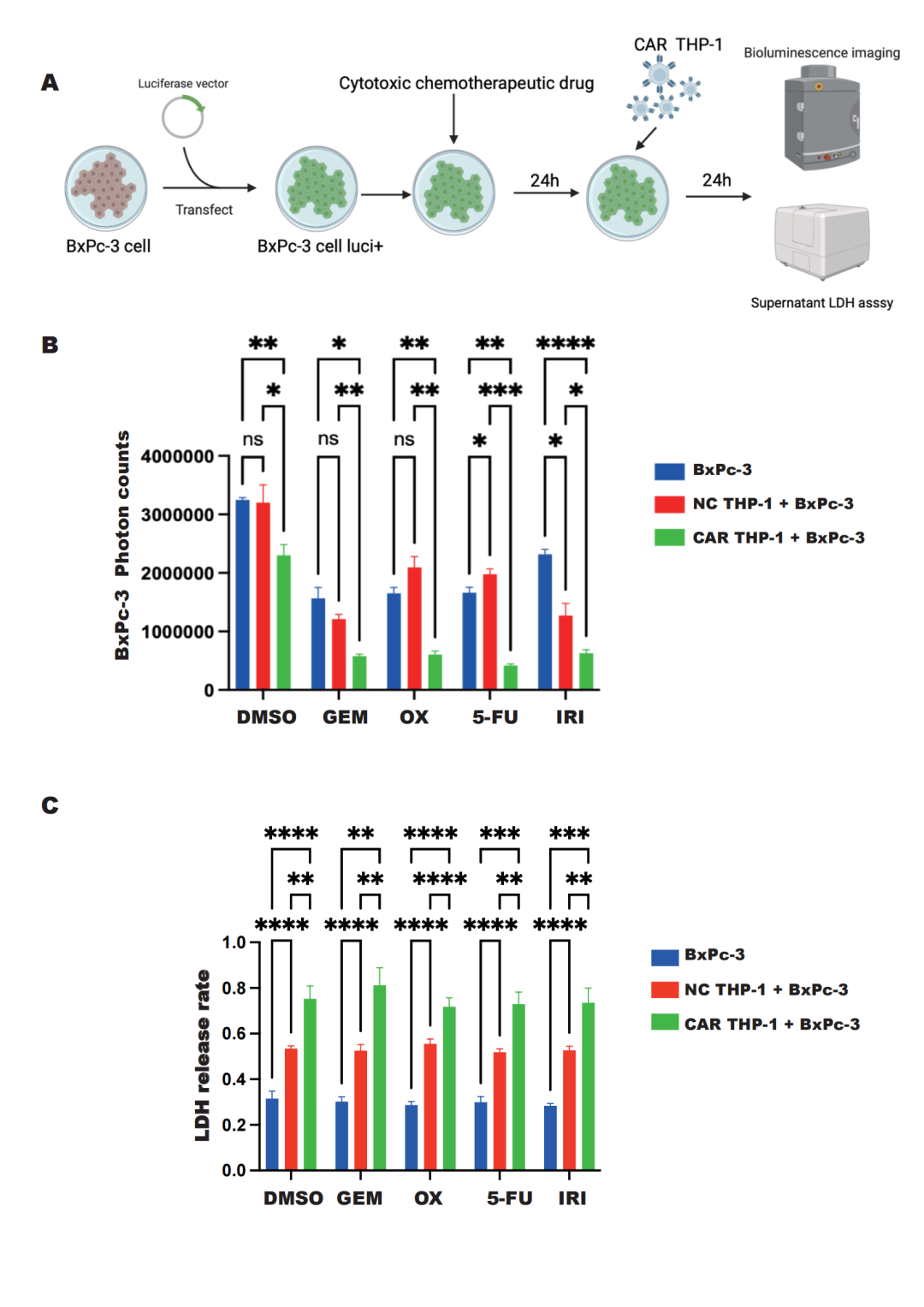
On December 6th, Professor Yin Xiushan's team from RocRock Biotech, in collaboration with Director Liu Qiaofei, Director Liao Quan, and Academician Zhao Yupei's team from Peking Union Medical College Hospital, jointly published a research paper titled "Chimeric antigen receptor macrophages targeting c-MET(CAR-M-c-MET) inhibit pancreatic cancer progression and improve cytotoxic chemotherapeutic efficacy" in the journal Molecular Cancer. RocRock Biotech is a co-corresponding author of the paper, with its founder Professor Yin Xiushan, along with Director Liu Qiaofei, Director Liao Quan, and Academician Zhao Yupei from Peking Union Medical College Hospital, serving as co-corresponding authors. Doctoral student Zheng Huaijin from Peking Union Medical College Hospital and Master's student Yang Xinzhe from Shenyang University of Chemical Technology are the co-first authors. This study has confirmed the high-efficacy anti-cancer action and safety of the therapy at both in vitro and in vivo levels, providing a solid scientific basis for the future conduct of clinical trials. In this research, scientists focused on the application of c-MET-targeting chimeric antigen receptor macrophages (CAR-M) in the treatment of pancreatic cancer. They observed that c-MET expression in pancreatic cancer tissue is significantly higher than in corresponding non-cancerous tissue, and this high expression is associated with a shorter patient survival period. Based on this, researchers developed a c-MET-targeting CAR-M cell (CAR-M-c-MET) using the human monocytic cell line THP-1. The modified macrophages showed highly specific recognition and binding capabilities against pancreatic cancer cells and demonstrated stronger phagocytic activity and cytotoxicity than normal macrophages. Moreover, the combination of CAR-M-c-MET cells with various chemotherapy drugs showed a synergistic enhancement in anti-tumor effects.
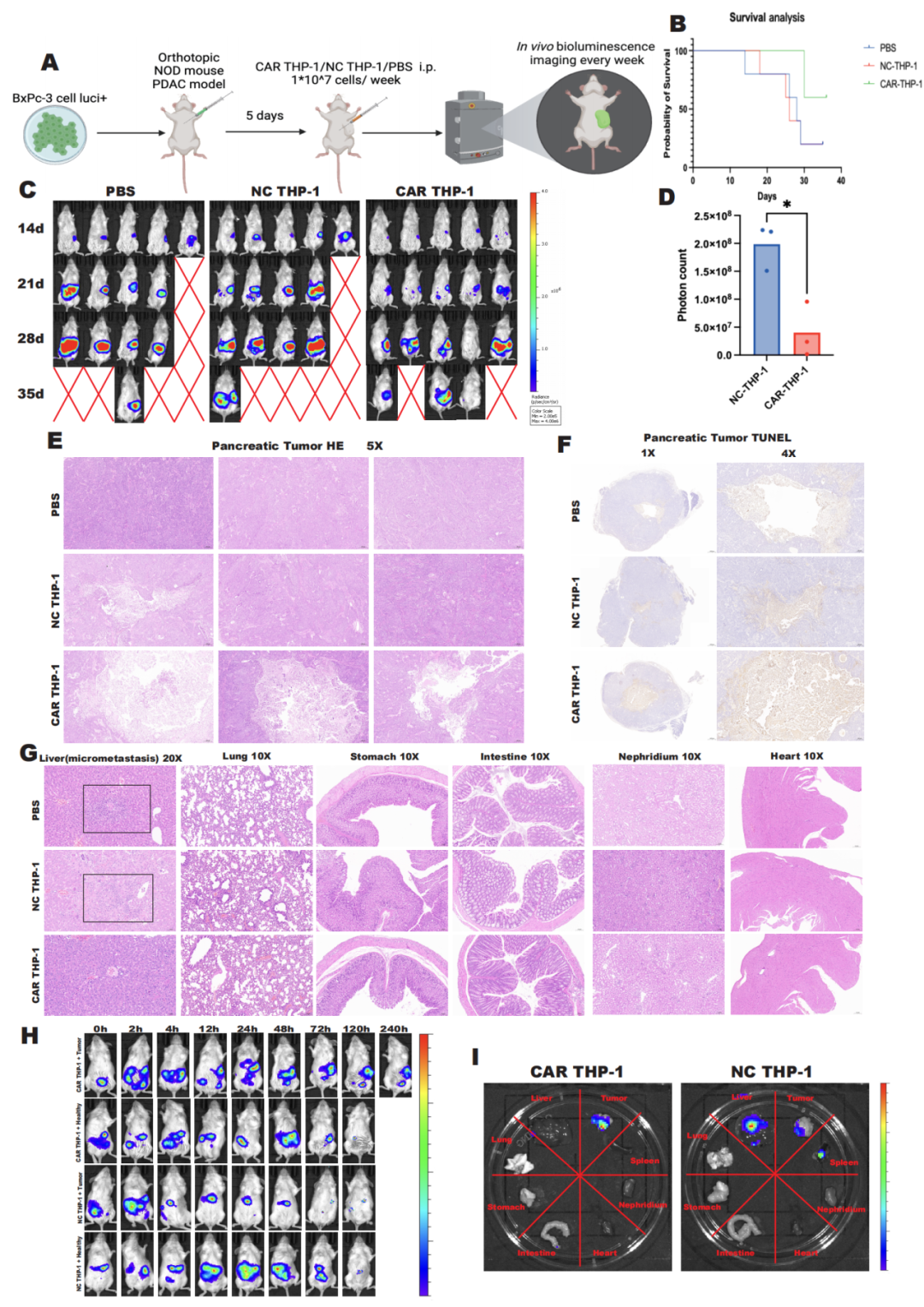
To validate the in vivo effects of CAR-M cells, researchers utilized a pancreatic cancer model in NOD/SCID mice and administered CAR-M-c-MET cells via intraperitoneal injection. They found that CAR-M-c-MET cells could rapidly home to the tumor region and effectively control tumor progression, with no significant adverse reactions observed throughout the process.
Further cytokine analysis and mRNA sequencing results indicated that CAR-M-c-MET cells produced higher levels of immune-activating factors compared to control macrophages. This study provides strong data support for the safety and efficacy of CAR-M cell therapy in the treatment of pancreatic cancer, confirming that CAR-M-c-MET not only effectively inhibits the progression of pancreatic cancer but also
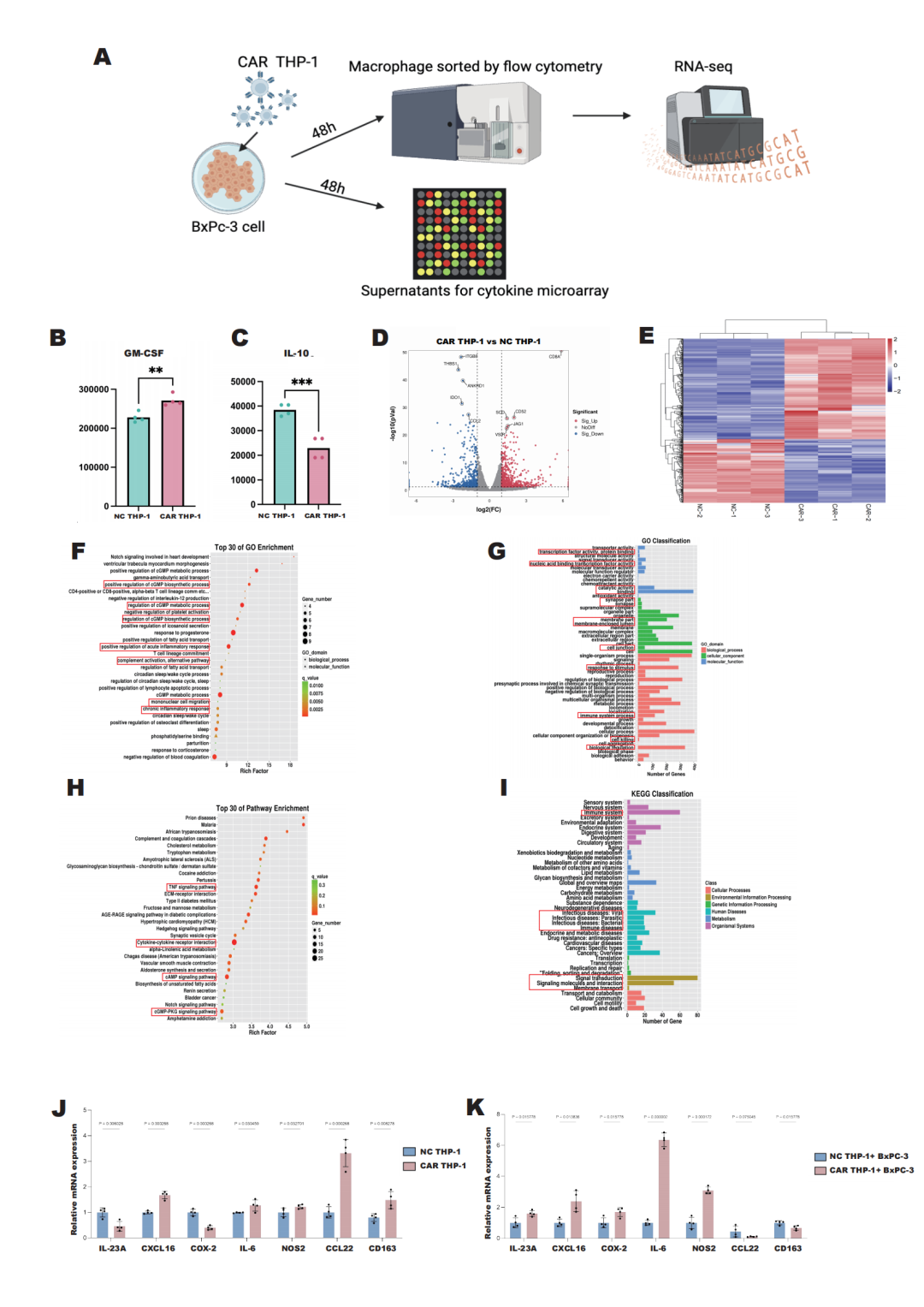
Conclusion: In this study, CAR-M-c-MET cells have demonstrated safety and efficacy in the treatment of pancreatic cancer in both in vitro and in vivo experiments. It has been confirmed that CAR-M-c-MET cells can not only effectively curb the progression of pancreatic cancer but also enhance the cytotoxic effects of chemotherapy drugs, with no significant toxic reactions observed in the experimental mice. Pancreatic cancer is the seventh leading cause of cancer-related deaths worldwide, with a mortality rate close to the incidence rate. The early diagnosis rate of pancreatic cancer is relatively low, at less than 5%, and about 60% of patients have metastasis at the time of their initial visit [1]. In 2020, there were 495,773 new cases and 466,003 deaths globally, with a five-year survival rate ranging from 9% to 11% [2], posing a serious threat to human health. According to data released by the National Cancer Center of the Chinese Academy of Medical Sciences, an estimated 134,374 new cases and 131,203 deaths from pancreatic cancer were expected in China in 2022, ranking eighth in incidence and sixth in mortality among all tumors [3]. Despite advances in pancreatic surgery and cytotoxic chemotherapy that have allowed more patients to safely undergo surgery and improved survival rates, the overall five-year survival rate for all patients remains below 10%, and it is only about 40% to 45% for localized pancreatic cancer without lymph node metastasis. Moreover, the response rate to the standard four-drug combination therapy (Folfirinox) is only about one-third. There is an urgent need for new treatment modalities to further improve this poor prognosis and treatment response.
[1] Owens DK, Davidson KW, Krist AH, et al. Screening for Pancreatic Cancer:US Preventive Services Task Force Reaffirmation RecommendationStatement. Jama.2019;322:438-444. doi: 10.1001/jama.2019.10232[2] Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer 」Clin.2022;72:7-33.doi: 10.3322/caac.21708.[3] Xia C, Dong X,Li H, et al. Cancer statistics in China and United States,2022: profiles, trends, and determinants. Chin Med J(Engl). 2022;135:584590.doi: 10.1097/cm9.0000000000002108.
0512-67998889(Suzhou)
18051093220(Shenzhen)

Cathy.Lv@rocrockbio.com(Suzhou)
lliangjing@rocrockbio.com (Shenzhen)