0512-67998889(Suzhou)
18051093220(Shenzhen)
RocRockBiotech Leads Innovation, Global Exclusive CAR-M Platform Achieves New Success in Liver Cancer Field!
RocRock Biotech, a pioneer in CAR-M cell therapy, has once again made significant strides in the field of liver cancer treatment with the recent publication of an article titled "GPC3-targeted CAR-M cells exhibit potent antitumor activity against hepatocellular carcinoma" in the international journal of cell biology, Biochemistry and Biophysics Reports. Professor Yin Xiushan, the founder of RocRock Biotech, served as the corresponding author of the paper. The latest global cancer research reveals that China has a high number of liver cancer patients globally. Facing the treatment of liver cancer and end-stage liver diseases, unprecedented challenges are being encountered. Although liver transplantation is currently considered the most effective means of treating end-stage liver disease, the shortage of donors, low matching rates, surgical complications, high costs, and immune rejection issues still limit its widespread use. In light of this, exploring innovative treatment strategies is particularly important and urgent.
The project was jointly tackled by RocRock Biotech in collaboration with Puheng Biotech and other units. In this study, our company constructed mouse macrophage cells RAW264.7 with CAR targeting GPC3 and explored its therapeutic potential in HCC. We designed a chimeric adenoviral vector (Ad5f35) that includes a CAR structure targeting GPC3, which comprises a scFv against GPC3 and the CD3ζ intracellular domain. It was introduced into RAW264.7 cells, and experiments were conducted using liver cancer cells with varying expression levels of GPC3 as target cells. The phagocytic and killing effects indicated that macrophages transduced with Ad5f35-GPC3-CAR (GPC3 CAR-Ms) exhibited antigen-specific phagocytosis and tumor cell clearance in vitro. GPC3 CAR-Ms demonstrated significant tumor-killing effects and promoted the expression of pro-inflammatory (M1) cytokines and chemokines. In a 3D tumor spheroid model of HCC, CAR-Ms were further proven to have significant tumor-killing effects.
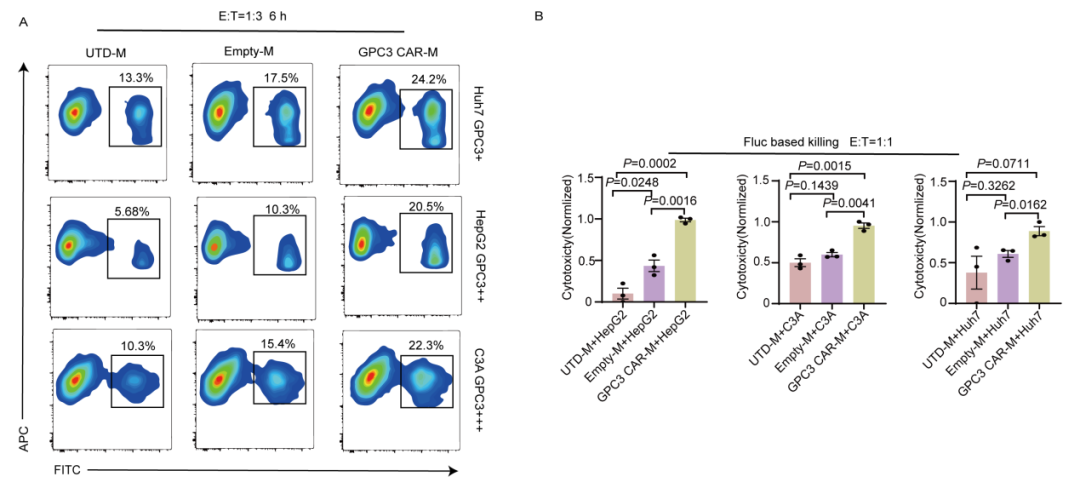
Figure 1: Assessment of Phagocytosis and Cytotoxicity The left panel shows the assessment of phagocytosis based on flow cytometry analysis, where target cells are labeled with the dye Celltracker Green. After co-culturing CAR-Ms with target cells for 6 hours, the proportion of CAR-Ms that have phagocytosed the control group. The right panel is based on the Firefly luciferase method, and the results indicate that CAR-Ms have significant cytotoxic capabilities.
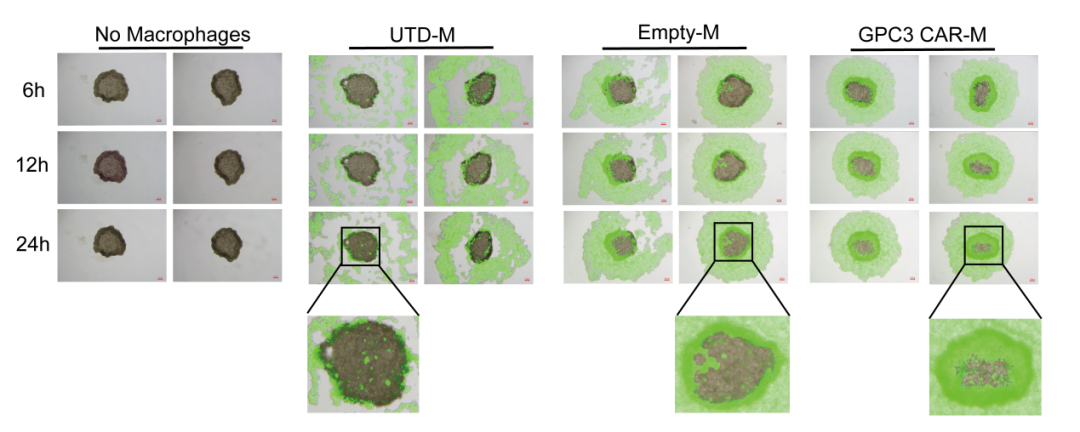
Figure 2: Targeting and Infiltration of CAR-Ms in the 3D Tumor Spheroid Model Cells labeled with the dye Celltracker Green, namely M/Empty-M/CAR-M cells, were respectively added to the 3D tumor spheroid model and photographed at 6h, 12h, and 24h. It was observed that the majority of CAR-Ms surrounded and infiltrated the 3D tumor spheroid model, indicating that CAR-Ms have a strong ability to target and infiltrate the 3D tumor spheroid (C3A spheroid) model. In summary, our research not only provides an innovative strategy for the treatment of liver cancer with GPC3-targeted CAR-Ms but also lays a solid foundation for the basic research and therapeutic advancements in liver cancer. It promotes the progress of clinical research on CAR-M cells.
0512-67998889(Suzhou)
18051093220(Shenzhen)

Cathy.Lv@rocrockbio.com(Suzhou)
lliangjing@rocrockbio.com (Shenzhen)