0512-67998889(Suzhou)
18051093220(Shenzhen)
Results Release | RocRock Biotechnology CAR-M Treatment for Prostate Cancer Preclinical Data Published
According to the predictions in "The Lancet Commission on prostate cancer: planning for the surge in cases," the number of prostate cancer patients worldwide is expected to increase from 1.4 million cases per year in 2020 to 2.9 million cases per year by 2040. It is estimated that the number of annual deaths from prostate cancer will increase by 85% over the next two decades, from 375,000 in 2020 to nearly 700,000 by 2040. Due to underdiagnosis in low- and middle-income countries and the omission of data collection, the actual numbers are likely to be much higher than recorded. Prostate cancer is one of the most common malignant tumors in the male urogenital system and has a severe impact on men's health and quality of life. Prostate cancer is considered an 'immunological desert,' as its unique tumor microenvironment makes immunotherapy less effective. Although traditional treatments such as surgery, radiotherapy, and androgen deprivation therapy show positive effects in the early stages, the treatment outcomes for advanced and metastatic prostate cancer are often unsatisfactory. Recently, the team of Professor Yin Xiushan from RocRock Biotechnology, in collaboration with Professor Liang Chao's team and Professor Zhang Ke's team from Southern University of Science and Technology, published a research paper titled "PSMA-specific CAR-engineered macrophages for therapy of prostate cancer" on the preprint website bioRixv. The paper described the construction of CAR-M cells targeting prostate cancer by introducing PSMA-specific single-chain variable fragments and costimulatory domains into macrophages, and verified their strong antitumor activity and safety in both in vitro and in vivo experiments, laying a solid preclinical foundation for the advancement of clinical trials.

CAR-M cells are genetically engineered to specifically recognize and kill tumor cells. In this study, researchers constructed PSMA-targeted CAR-M cells, which were transduced with a CAR construct containing PSMA-specific single-chain variable fragments (scFv) and costimulatory domains, achieving a specific attack on prostate cancer cells. In vitro experiments confirmed that CAR-M cells could specifically recognize and kill PSMA-expressing prostate cancer cells, and they exhibited a strong pro-inflammatory phenotype, providing a new approach for the immunotherapy of prostate cancer.
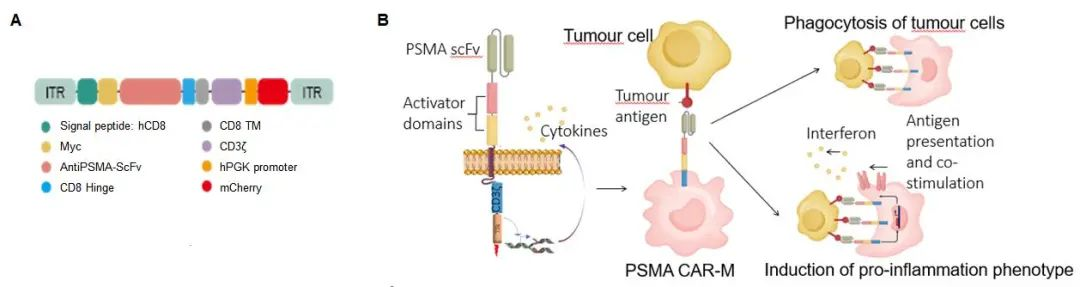
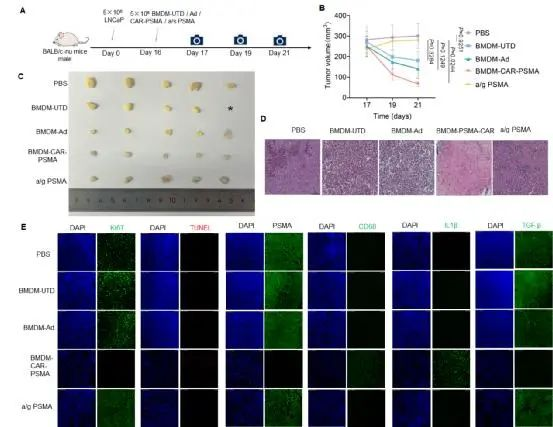
To verify the effect of CAR-M cells in vivo, researchers injected PSMA-targeted CAR-M cells into a xenograft mouse model. The experimental results showed that the tumor volume in the mice treated with CAR-M cells was significantly reduced, and the proportion of cell apoptosis in the tumor tissue was markedly increased. Immunohistochemical analysis revealed that CAR-M cells could effectively infiltrate tumor tissues, and no significant off-target toxicity was observed. These results confirmed the antitumor effects of PSMA-targeted CAR-M cells in vivo and supported their potential as a novel immunotherapy for prostate cancer.
Source:
doi: https://doi.org/10.1101/2024.09.07.611792
0512-67998889(Suzhou)
18051093220(Shenzhen)

Cathy.Lv@rocrockbio.com(Suzhou)
lliangjing@rocrockbio.com (Shenzhen)