
Recently, at the special invitation of the CELL series magazine "STAR Protocol," RocRock Biotechnology, as the leading unit, published an article titled "Protocol for generating of human CAR-engineered macrophages by Vpx-containing lentivirus," which outlines the clinical-grade macrophage CAR transfection technology and process specifications. This technological process significantly improves the transfection efficiency of human macrophages to over 95%, marking the company's recognition by authoritative institutions in the field of CAR-M preparation. It also demonstrates RocRock's strength in technological innovation, providing an important reference for the research and application of global CAR-M therapies. Since its establishment, RocRock Biotechnology has been committed to the research and development and innovation of biomedical technologies, accumulating rich experience and technical reserves, especially in the field of cell therapy. This platform has laid a solid technical foundation for RocRock's systematic cooperation in chronic diseases such as pulmonary fibrosis, liver cirrhosis, and arthritis obesity. Globally, for patients with various types of solid tumors, existing treatment methods often fail to achieve the desired therapeutic effects. Even revolutionary drugs like immune checkpoint inhibitors such as PD-1/PD-L1 antibodies are only effective for some patients in certain cases, and their overall response rates are not always high. This means that a large number of solid tumor patients have not benefited lastingly from these innovative therapies. Therefore, CAR-M therapy, as a new type of immunotherapy, has attracted widespread attention for its unique targeting and multifunctionality. However, due to the innate resistance of human macrophages to HIV-1-based lentiviral vectors, traditional gene-editing technologies have difficulty achieving efficient gene transduction in macrophages. RocRock Biotechnology has solved this technical challenge by introducing Vpx, an auxiliary protein from HIV-2 or SIV. Vpx can specifically degrade the SAMHD1 protein within macrophages, thereby greatly improving the infection efficiency of lentiviral vectors in macrophages. The process specifications published by RocRock cover the entire process from cell isolation, culture, to lentiviral packaging and infection, providing researchers with a complete set of CAR-M preparation guidelines.
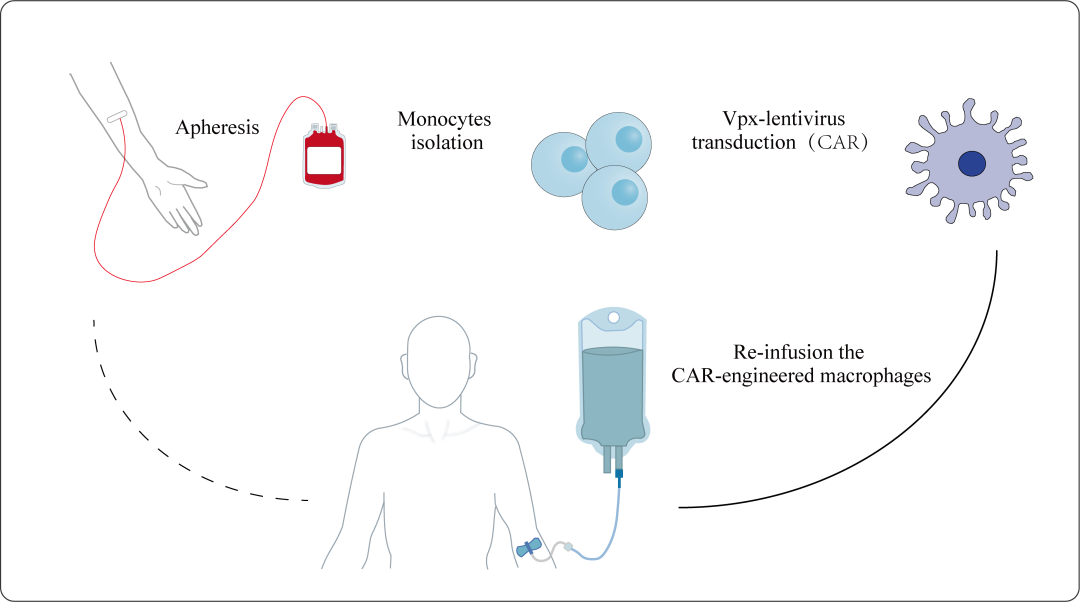
RocRock Biotechnology has demonstrated tremendous potential in CAR-M preparation, which has been preliminarily validated through seven successful clinical trials. Despite the significant achievements, RocRock Biotechnology is not complacent. The company will continue to deepen cooperation with domestic and international research institutions, constantly explore and perfect CAR-M preparation technology, and strive to bring more effective treatment options to patients in the future. At the same time, RocRock will also actively fulfill its social responsibilities, promote the benefits of scientific and technological achievements to a broader population, and contribute to the cause of human health.


